Almego – Intelligent SDS Authoring Software by EcoOnline
Take control and simplify the creation of your SDSs with SDS Authoring software and expert guidance.
Convenient, fast, and correct SDSs
Produce compliant SDSs in up to 80 markets in under 2 minutes.
Easily meet compliance
Simplify the creation of high-quality SDSs that are compliant in over 80 countries.
Strong integrations
Almego has built-in migration tools, a cutting-edge API and direct access to ECHA.
“I really find this a great and easy user-friendly system, easy to teach new users.”
Bill D
Senior Site Manager
“User friendly Chemical Inventory list with easy access to SDS.”
Remy H
Senior Engineer
“This is a must for any business.”
Mandy H
Health and Safety Manager
Turn audit stress into peace of mind
with automation
Make sure that your SDSs are compliant and audit-proof with our SDS authoring software.
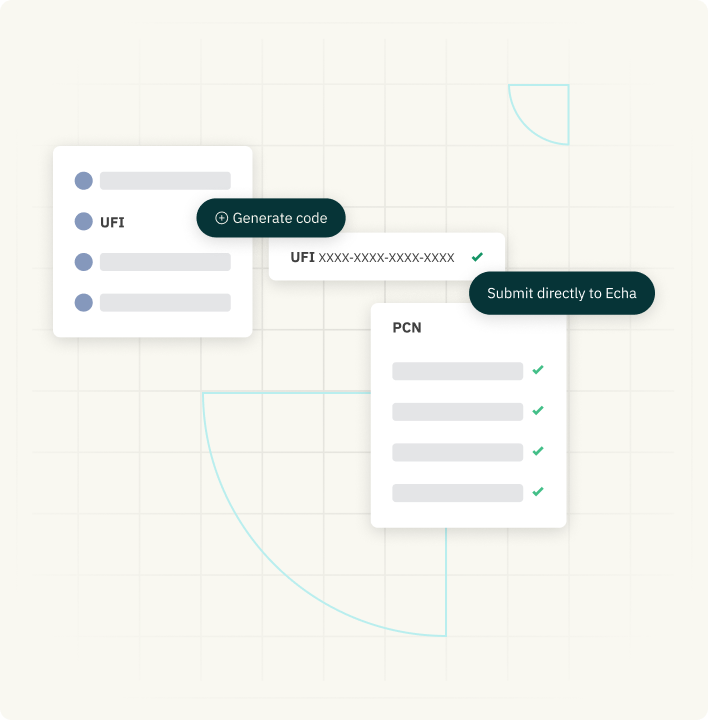
UFI Code & PCN Submission
Generate Unique Formula Identifier codes (UFI) and submit them to the Poison Centre Notification (PCN) system directly.
Features that streamline SDS authoring
SDS authoring often means sinking constant hours into tracking legislation and chemical data changes to ensure compliant SDSs. Choose SDS authoring software that’s ISO 9001 certified for a reason and start creating better SDSs, faster.

Languages
Access 80+ SDS country/language versions.

Expert guidance
Built-in guidance functionality via chat, phone, and email support.

Change management
Supports compliance by automatically identifying changes to relevant legislation and automating change management using a simple workflow.

Effective onboarding
Speed up onboarding with our built-in integrations.

Stand out from the competition
Almego’s design module makes it easy to be consistent with your organisation’s branding.

Clear and understandable reports on SDS data
Use Almego’s reporting tool to retrieve and report on different topics, e.g. problematic substances, transport data, white label data.
SDS Smart Extraction

State of the art LLMs
SDS Smart Extraction combines 20+ years of experience built into the SDS Authoring Software with state of the art Large Language Models.

Smart extraction of SDS data
SDS Smart Extraction pulls data points from PDF versions of SDSs and enables users to replicate or continue editing PDF versions of SDSs in Almego.

Unprecedented accuracy
Extracted data has unprecedented accuracy, exceeding human accuracy for important but repetitive tasks.

Automatic translations
Automatic translation of extracted text in up to 39 languages.
“With the ever-changing landscape of regulatory and compliance, using this system has enabled our company to stay current and up to date.”
Adrian Rodriguez
Vice President of Quality and Safety, Mark Cuban Cost Plus Drug Company


Free up hours of admin time with 20+ years of SDS expertise
Benefit from automation for your SDS authoring with an affordable, usage-based price model. When you schedule a chat with one of our in-house experts, you’ll see how easy the process is when you have SDS Authoring Software.
Be in the know
Access industry-leading information
FAQs
- A user-friendly and intuitive interface
- Built-in guidance and help functionalities
- A high degree of automation with smart-rule authoring
- Easy updating and translations to many countries/languages
- A trustworthy CLP calculator
- Easy data migration from existing software
- Easy integration with other software
- A price model that is tailored with flexible terms
This is exactly what we have developed with ALMEGO®. This new SDS authoring software has been designed with a focus on the needs of users. Delivered in an up-to-date SaaS platform.
ALMEGO® SDS authoring software is priced based on usage. You simply pay a yearly fee based on usage of your ALMEGO® SDS database. The yearly fee includes all updates.
The main objective of a safety data sheet is to convey comprehensive information about a substance or mixture for use in workplace chemical control regulatory frameworks. Both employers and workers use it as a source of information about hazards, including environmental hazards, and to obtain advice on safety precautions.
The SDS is product related and usually not able to provide specific information that is relevant for any given workplace where the product may finally be used Although where products have specialized end uses the SDS information may be more worker specific. The information therefore enables the employer to:
- develop an active programme of worker protection measures, including training, which is specific to the individual workplace
- to consider any measures which may be necessary to protect the environment.
In addition, the SDS provides an important source of information for other target audiences. So certain elements of information may be used by those involved with the transport of dangerous goods, emergency responders (including poison centres), those involved in the professional use of pesticides and consumers.
However, these audiences receive additional information from a variety of other sources such as the UN Recommendations on the Transport of Dangerous Goods, Model Regulations and package inserts for consumers and will continue to do so.
You need to provide a SDS if:
1. You supply:
(a) a substance or a mixture that is classified as hazardous under the CLP Regulation
or
(b) a substance that is persistent, bioaccumulative and toxic (PBT), or very persistent and very bioaccumulative (vPvB)
or
(c) a substance that is identified as having endocrine disrupting properties
or
(d) a substance that is included in the European Chemicals Agency’s ‘Candidate List’ of substances of very high concern (SVHC)
2. You are a supplier and your customer requests an SDS for a mixture that is not classified as hazardous under the CLP Regulation, but contains either:
(a) a substance posing human health or environmental hazards in a concentration of ≥ 1 % w/w (solids or liquids) or ≥ 0.2 % v/v (gases)
or
(b) a substance that is:
- carcinogenic in category 2
- toxic to reproduction in category 1A, 1B and 2
- a skin sensitiser category 1
- a respiratory sensitiser category 1
- or has effects on or via lactation
- or is persistent, bioaccumulative and toxic (PBT)
- or very persistent and very bioaccumulative (vPvB) in an individual concentration of ≥ 0.1 % w/w
or
(c) a substance on the ‘Candidate List’ in an individual concentration of ≥ 0.1 % w/w
or
(d) a substance for which there are Europe-wide workplace exposure limits or have relevant national workplace exposure limit values in the country you supply your product.
3. You are a supplier of a product listed as a ‘special case’ Annex 1 of the CLP Regulation for which there are labelling derogations; e.g., gas containers intended for propane, butane or liquefied petroleum gas.
Find below some of the most normal situations where you do not need to provide an SDS:
1. If the substances/mixtures are supplied in the EU and isn’t classified as hazardous or considered PBT, vPvB or of equivalent concern (e.g., endocrine disruptors) and does not contain hazardous substances in section 3 above their cut-off limit.
2. For certain products intended for the final user, e.g. medicinal products or cosmetics.
3. If you offer or sell dangerous substances or mixtures to the general public and you provide sufficient information to enable users to take the necessary measures as regards safety and the protection of human health and the environment.
OHS is an acronym for Occupational Health and Safety. Occupational health and safety software, like EHS Software keeps a record of health and safety data and allows you to generate analysis and trends reports to ensure your employees are covered in terms of Health and Safety.
According to the REACH Regulation, the safety data sheet (SDS) shall be supplied in the official language(s) of the Member State where the substance or mixture is placed on the market, unless the Member State concerned provide otherwise.
This also applies to exposure scenarios, which are a part of an SDS. A document listing the languages required for safety data sheets and labels within the EU is available here.
Yes, the SDS format can be used. Suppliers, who do not have to supply an SDS may be obliged to provide certain information in accordance with the REACH Regulation or they may choose to provide an SDS on a voluntary basis.
The suppliers of a substance or a mixture may also choose to provide information in the safety data sheet format even if they are not obliged to provide any information under Articles 31 or 32 under REACH. In this case, they should also clarify that the SDS is not provided pursuant to Article 31 of REACH, and then explain why they provide it.
Among possible solutions would be the addition to the relevant SDS of a phrase such as ‘A safety data sheet is not required for this product under Article 31 of REACH’.
The SDS should be provided to the recipient free-of-charge, on paper or electronically, e.g. by postal delivery, fax or email. A system that merely requires customers to obtain a SDS from a company’s website is not allowed. An SDS should be provided either before or at the time of first delivery of the substance or mixture.
Where a customer re-orders substances or mixtures, then the supplier does not need to re-supply the SDS, unless the contents have changed significantly.
If there is no basis for changing the information in the SDS there is no expiry date. A version 1.0 of the SDS can therefore exist infinitely as long as the contents in the SDS remain accurate and up to date.
If the revised contents result in a major change of the SDS then the REACH regulation requires that the supplier of the SDS provides an updated version of the SDS to all recipients to whom the substance or mixture has been supplied within the preceding 12 months.
The supplier is furthermore required to inform the recipient about the where the SDS has changed. To allow the recipient to understand and trace the different versions of the changed SDS’s the supplier shall give information about the changes in either section 16 or elsewhere in the SDS.
To allow the recipient to distinguish between the different versions of the SDS it is suggested that an incremental numbering system be used to identify new versions of an SDS. In such a system, changes to versions could be identified by an increment by an integer e.g.:
Version 1.0: initial issue
Version 2.0: first change requiring provision of update to former recipients.
A change to the SDS is considered major when:
- new information which may affect the risk management measures, or new information on hazards becomes available;
- an authorisation has been granted or refused
- a restriction has been imposed
There are no specific recommendations on when a change in an SDS is considered a “major” or a “minor” change, thus it is a matter of interpretation to decide whether the change does or does not affect the understanding of the risk from using the product and/or affects the measures needed to control the risk. As a rule of thumb any change to the following sections in the SDS would normally mean that the change is considered major:
Section 1
- Trade name
- Uses advised against
- Contact person
- Emergency phone number
Section 2
- Classification of the substance or mixture
- Hazard pictograms
- Signal word
- Hazard statements
- Precautionary statements
- Hazardous substances
- Additional labelling
Section 3
- Changes in the information about the components in the products whether the product is a substance or a mixture
Section 8
- Occupational exposure limits
- DNEL and/or PNEC values
- Recommendations for respiratory protection
- Recommendations for skin protection
- Recommendations for hand protection
- Recommendations for eye protection
Section 12
- The result of the PBT/vPvB assessment
Section 14
- Transport information in relation to ADR, IMDG, ICAO, RID and/or ADN
Section 15
- Restrictions of use
- Demands for specific education
No. In the requirements to the SDS set by the REACH regulation it is specifically pointed out that the safety data sheet shall be prepared by a competent person, who shall consider the specific needs and knowledge of the user audience, as far as they are known. Suppliers of substances and mixtures shall ensure that such competent persons have received appropriate training, including refresher training.
In this context a “competent person” means a person (or combination of persons), who has or have, because of their training, experience and continued education, sufficient knowledge for the compilation of the respective sections of the SDS or of the entire SDS.
The supplier of the SDS can delegate this function to his own staff or to third parties. It is not necessary that the expert knowledge be provided in full by one single competent person as it is understood that a single person rarely has extensive knowledge in all the fields covered by an SDS. It is thus necessary that the competent person rely upon additional competences, either internal or external. The competent person should ensure the consistency of the SDS, especially if he acts as the coordinator of a group of people.
No. The information that is required to appear in an SDS cannot be claimed as confidential. This includes information about the substances and their amount in the product. It is however allowed to use weight ranges of percentages to indicate the amount.
When using a range of percentages, the health and environmental hazards shall describe the effects of the highest concentration of each ingredient. It is not allowed not to indicate identification parameters such as CAS numbers, EC numbers and REACH numbers if they exist.
Nothing. According to the REACH regulation, the SDS and any required updates to it must be provided free of charge.
No. There may be places in the SDS where information will not be completed because of e.g. a data gap, or application can be questioned, etc. However, the SDS must contain an explanation or a justification of why the section has not been completed.