Improve, Edit, & Submit
Get it ALL Done With ALMEGO®
Save time with next-generation SDS authoring software that enables you to generate Unique Formula Identifier codes (UFI) and submit them to the Poison Centre Notification (PCN) system directly.
“The ALMEGO® system gave us an easy way to produce SDSs with different trade names and in many different languages.”
Inga Göransson, QES Manager, SimFas
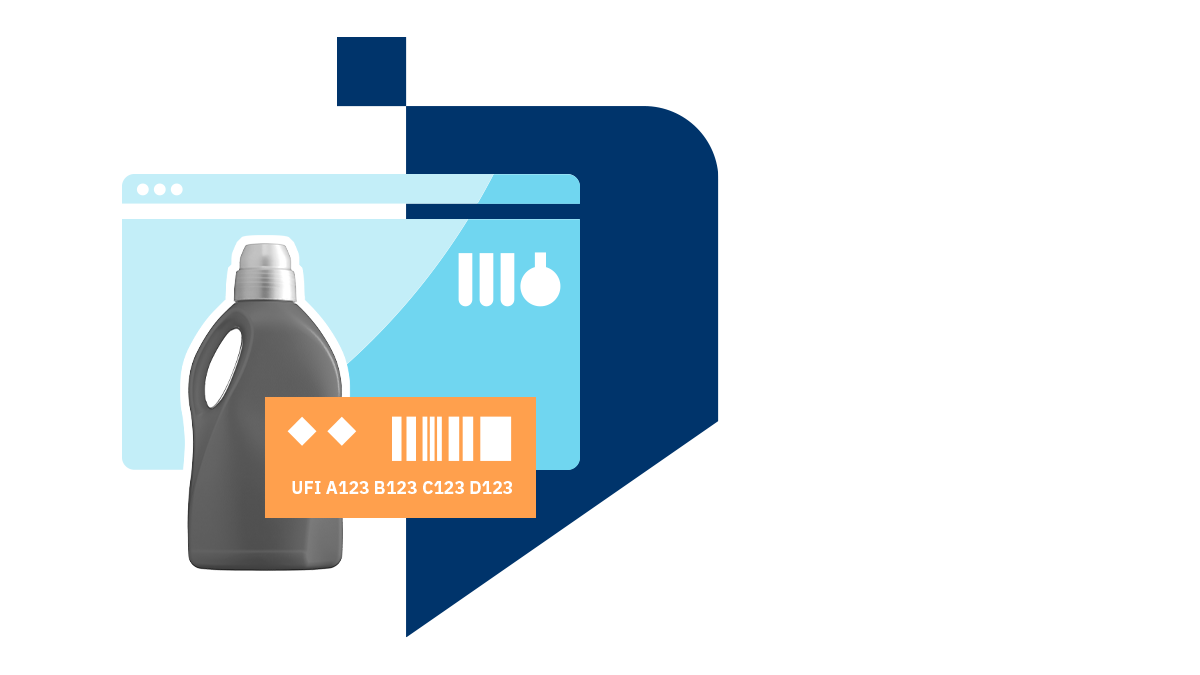
Save time with powerful software.
UFI codes and submission of PCN dossiers are the centre of attention for chemical companies in Europe right now. Generate and submit new information without stress.

One submission for each product.
Complete PCN submissions in minutes
When you’re able to submit your PCN directly to the European Chemicals Agency (EHCA) API, you can receive direct feedback about your submission and correct any deficiencies. Re-submit your submission for approval without any added hassle. Our software makes this process so quick and simple that you’ll start to forget how frustrating it used to be.
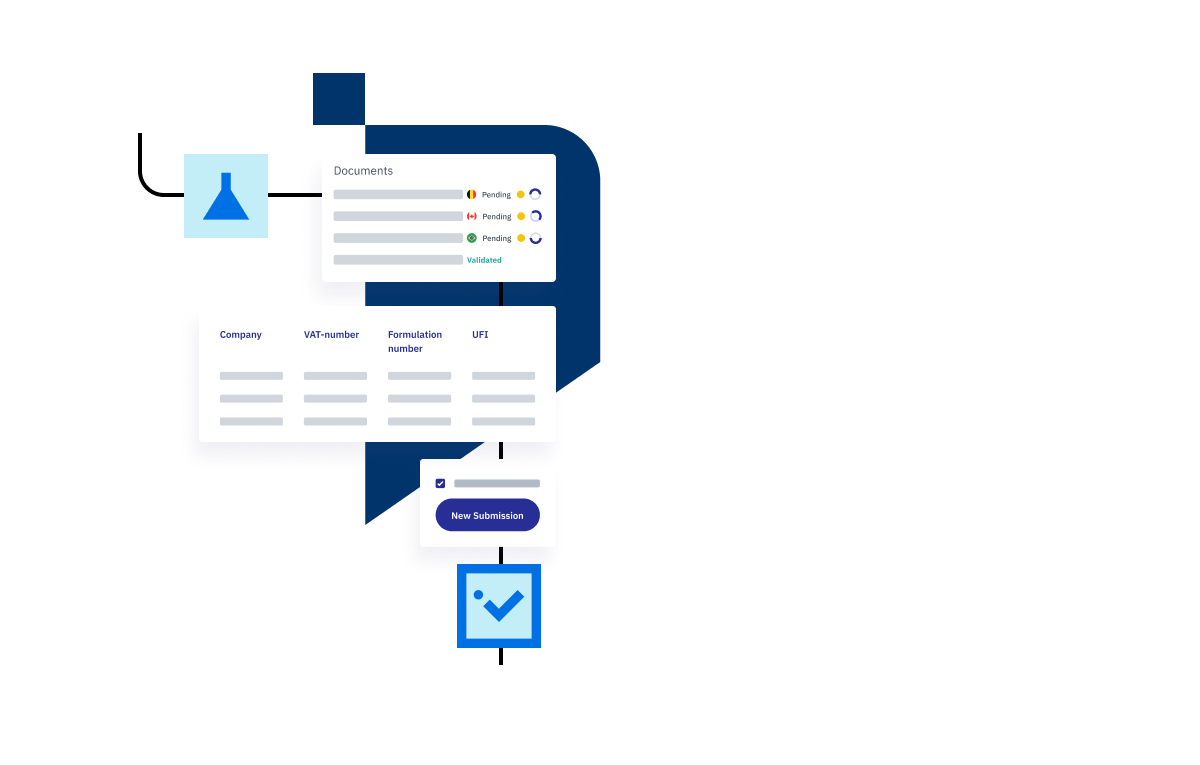
- Communicate directly with the European Chemicals Agency (EHCA) API.
- Automatically compile data for PCN submission from multiple countries and languages at the same time.
- Clear submission validation from EHCA.
Getting started is simple

Fill out the contact form
We’ll be in touch in the next 24-48 hours on weekdays to schedule a chat with one of our consultants.
Your personalised guided tour
You’ll get concierge-level service, with a customised tour to demonstrate how our software can help.

Start your journey towards a safer workplace
Discover how simple EHS can be when you’ve found the right solution for your organisation.
“We have been using ALMEGO® now for a period of time and find the navigation and creation of Safety Data Sheets an easy process with this software. I also find the help I received at times of uncertainty was top class and prompt with all replies giving me fantastic service.”
Pearl McCartney, Nicobrand Ltd.
Frequently asked questions
What is UFI?
In addition to the UFI, you are also required to submit certain information to the national poison centres on your product. This includes composition, trade name, colour, packaging, product category and toxicological information.
The UFI aims to establish an unambiguous link between the information you provide with the product you place on the market. The condition for assigning a UFI is that all products labelled and notified with the same UFI need to share the same composition.
How will UFI be used?
What do you need to create a UFI?
Most likely your company already uses internal formulation codes. If they are numerical only – between 0 and 268 435 255 – you can use them directly in the UFI Generator. In other cases, such as when they are alphanumeric or contain other characters, you will need to first assign new formulation numbers to your mixtures that follow the required format.
It is essential that you do not reuse the same formulation number using the same VAT number when the mixtures have different compositions.
When is UFI implemented?
Whether you are making a new notification or updating an existing one, you will have to make a notification in accordance with Annex VIII before placing the product on the market after 1 January 2021 (consumer use and professional use) or 1 January 2024 (industrial use only).
A notification must be submitted in each Member State where you intend to place your product on the market.
Discover more ways Almego® simplifies safety management
When you speak with one of our in-house experts, you get to see exactly how Almego® software can work within your current processes to help your organisation grow a positive safety culture.
Get your questions answered

Trusted by 10,000+ customers worldwide
With the knowledge from 90 different industries we have developed our platform to make sure it tailors to your needs.
Don't take our word for it.




Save more time with one convenient platform
Make better decisions faster with flexible modules that give you the exact information you need when you need it.
Reduce time-consuming health and safety training paperwork with a customisable digital learning platform.
Get a clear picture of your environmental performance data with tools that make it easier to turn insights into positive actions.


